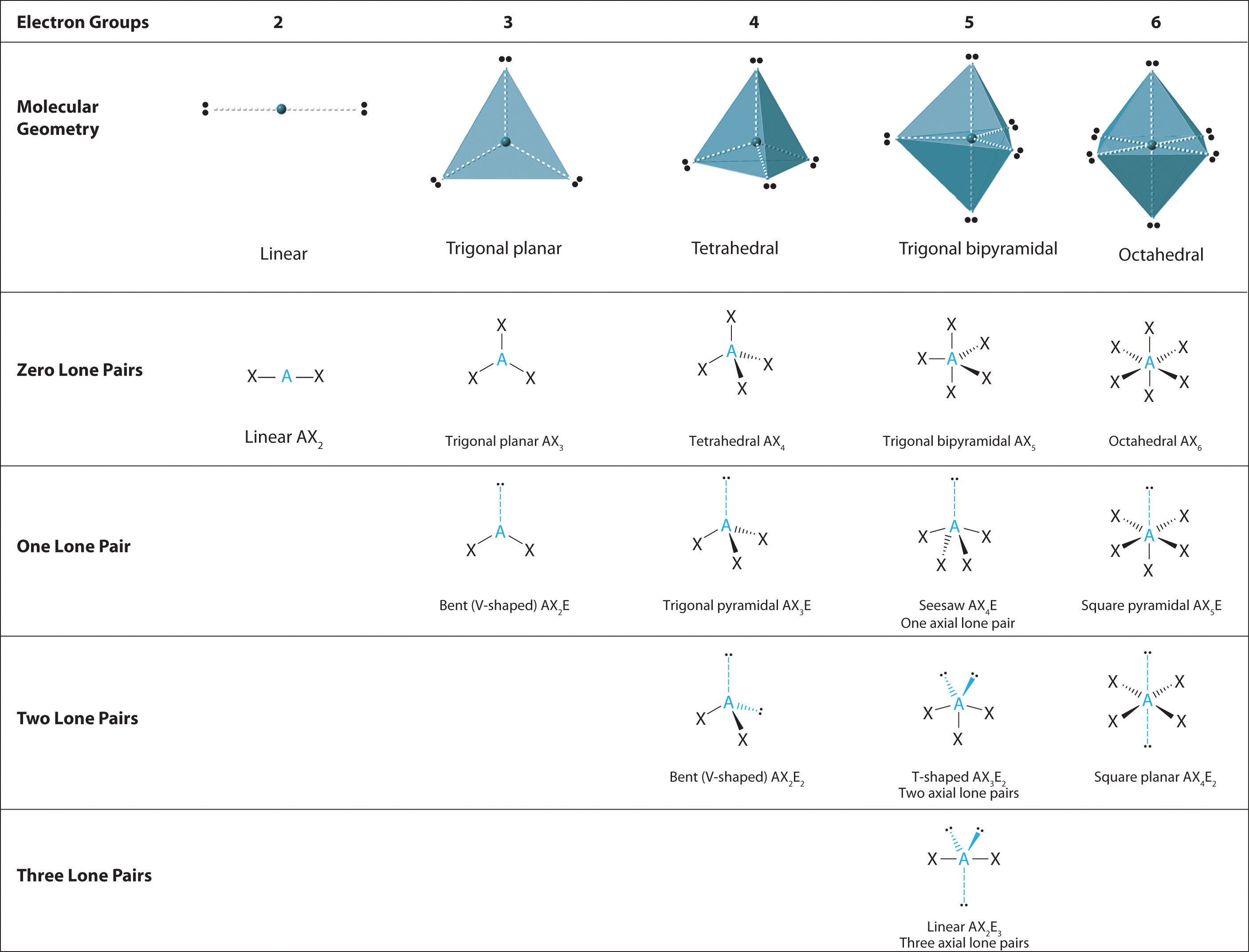
These diagrams tell us that the f 2 molecule has a single bond, the co 2 molecule has two double bonds, and the hcn molecule has one single bond plus one triple bond. Also state whether the neutral molecules are polar or nonpolar.
Draw A 3 Dimensional Sketch Each Of The Molecule Or Ions, Learn this topic by watching 3d hybrid orbital drawings concept videos. It also has the least electronegativity w/c allows electrons to be drawn out farther thus, lowering the repulsions. The relative arrangement of atoms is shown in the box.
Determine the geometry about each interior atom in each molecule and sketch the molecule. 3 molecule and the shape of the bf €ion. $$ \mathrm { pi } _ { 3 } ^ { 2 } $$ , c. First of all, we need to calculate the total number of valence electrons present in hydronium ion.
Answered 1. Draw the complete Lewis Structure… bartleby
Rules for constructing molecules with the model kit 1. The three dimensional shape or configuration of a molecule is an important characteristic. For each of the following molecules, draw a lewis structure and predict the molecular geometry, hybridization, overall polarity, and imfs of the molecule. Rules for constructing molecules with the model kit 1. Molecular models & lewis dot structures objectives: (b) name the type of hybrid orbitals that the central atom forms.

Draw A Threedimensional Structure For Each Compou, Part a draw the lewis structures for each of the following ions and molecules. $$ \mathrm { nh } _ { 2 } \mathrm { cl } $$ , e. Refer to the inside of the container lid for the. Now we have found the center atom and sketch of nf 3 molecule. For each of the following molecules, draw.

Molecular Geometry and Molecular Polarity Chemists, $$ \mathrm { cl } _ { 2 } \mathrm { o } $$ , d. Now we have found the center atom and sketch of nf 3 molecule. Part a draw the lewis structures for each of the following ions and molecules. Rules for constructing molecules with the model kit 1. (b) name the type of hybrid orbitals that.
Predicting and Drawing Molecular Geometry and Shape, Learn this topic by watching 3d hybrid orbital drawings concept videos. Molecular models & lewis dot structures objectives: 3 (e) is the molecule polar or nonpolar? Name the type of bond formed in this reaction. Our primary goal for today is to be able to draw diagrams of molecules, such as we see in figure 1.
Media Portfolio, Ch3co2ch3 (h3ccooch3 one o atom attached to 2nd c atom; Draw the electron dot structure. Xeo3 lewis structure sketch shape hybridization b. Nh2co2h (h2ncooh both o atoms attached to c) Each colored ball in your kit corresponds to different atom or different group of atoms.

Solved 1.49 Draw A Threedimensional Representation For E, Also state whether the neutral molecules are polar or nonpolar. Molecular models & lewis dot structures objectives: Three dimensional configurations are best viewed with the aid of models. $$ \mathrm { pi } _ { 3 } ^ { 2 } $$ , c. (a) draw the lewis structure of h 2o 2.
Solved I Don�t Know If I�m Right. Draw A 3dimensional Sk, Our primary goal for today is to be able to draw diagrams of molecules, such as we see in figure 1. There are two lone pairs of electron. Let’s try to draw the lewis structure of h3o+. Draw the lewis dot structure for each of the following polyatomic ions: $$ \mathrm { pi } _ { 3 } ^ {.

Draw A Threedimensional Structure For Each Compou, Between other oxygen atoms, there are only single bonds with phosphorous atom. The three dimensional shape or configuration of a molecule is an important characteristic. Brf3 lewis structure sketch shape hybridization For the following molecules or ions (where the central atom is underlined): Xeo3 lewis structure sketch shape hybridization b.

This figure shows the structure of a water molecule. The, Part a draw the lewis structures for each of the following ions and molecules. Remember that, there are total of thirteen electron pairs. Each colored ball in your kit corresponds to different atom or different group of atoms. 2009 test #1, problem #3 answer the following questions about the difluoroiodate ion (if2 +). First of all, we need to calculate.
Solved I Don�t Know If I�m Right. Draw A 3dimensional Sk, Draw sketches to predict the shape of a cif� ion and the shape of a clf5 molecule. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond. Show all atoms and bonds between them. Sketch a reasonably detailed picture of this model on your report form. Name.

Chapter 5.1 Predicting the Geometry of Molecules, Determine the shape of the molecule. Name the type of bond formed in this reaction. Learn this topic by watching 3d hybrid orbital drawings concept videos. The relative arrangement of atoms is shown in the box. The three dimensional shape or configuration of a molecule is an important characteristic.

Note that we do not actually needto draw all the resonance, + sign indicates losing an electron from the total valence electrons. Remember that, there are total of thirteen electron pairs. Three dimensional configurations are best viewed with the aid of models. The relative arrangement of atoms is shown in the box. 3 (e) is the molecule polar or nonpolar?

Which Of The Following Represents The Lewis Dot Diagram Of, $$ \mathrm { nh } _ { 2 } \mathrm { cl } $$ , e. These diagrams tell us that the f 2 molecule has a single bond, the co 2 molecule has two double bonds, and the hcn molecule has one single bond plus one triple bond. Refer to the inside of the container lid for the. Now,.
Drawing Organic Molecules Organic Chemistry Help, Draw the electron dot structure. Nh2co2h (h2ncooh both o atoms attached to c) Draw lewis structures for atoms, ions and simple molecules. Hybridization, bond angles and polarity draw lewis dot structures in your notebook for pcls, cio2 , sibr4, of2, and bros (structures obeying the octet rule). Refer to the inside of the container lid for the.

9.7 The Shapes of Molecules Chemistry LibreTexts, Let’s try to draw the lewis structure of h3o+. Each colored ball in your kit corresponds to different atom or different group of atoms. Also state whether the neutral molecules are polar or nonpolar. Also state whether the neutral molecules are polar or nonpolar. Rules for constructing molecules with the model kit 1.

Draw A Threedimensional Structure For Each Compou, + sign indicates losing an electron from the total valence electrons. In each case, name the shape. Determine the approximate bond angles. Now the important point is, not to forget about the + sign. Our primary goal for today is to be able to draw diagrams of molecules, such as we see in figure 1.

Answered 1. Draw the complete Lewis Structure… bartleby, Also state whether the neutral molecules are polar or nonpolar. 2009 test #1, problem #3 answer the following questions about the difluoroiodate ion (if2 +). Three dimensional configurations are best viewed with the aid of models. Determine the approximate bond angles. These diagrams tell us that the f 2 molecule has a single bond, the co 2 molecule has two.

Molecular shape Atomic combinations Siyavula, The relative arrangement of atoms is shown in the box. 3 3 (c) name the geometry of the electron distribution about the oxygen atoms. The three dimensional shape or configuration of a molecule is an important characteristic. In each case, name the shape. Refer to the inside of the container lid for the.

Write resonance structures for CO32 and HCO3 ions class 12, This shape is dependent on the preferred spatial orientation of covalent bonds to atoms having two or more bonding partners. For each of the following molecules, draw a lewis structure and predict the molecular geometry, hybridization, overall polarity, and imfs of the molecule. Water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond.

What is the molecular geometry about nitro… Clutch Prep, (b) name the type of hybrid orbitals that the central atom forms. It also has the least electronegativity w/c allows electrons to be drawn out farther thus, lowering the repulsions. The following geometries may be. Ch3co2ch3 (h3ccooch3 one o atom attached to 2nd c atom; Draw sketches to predict the shape of a cif� ion and the shape of a.

Solved Draw A Lewis Structure For Each Of The Following M, Refer to the inside of the container lid for the. Draw lewis structures for atoms, ions and simple molecules. Also state whether the neutral molecules are polar or nonpolar. $$ \mathrm { pi } _ { 3 } ^ { 2 } $$ , c. $$ \mathrm { scl } _ { 2 } $$ , b.

2.6 Molecules and Molecular Compounds Chemistry LibreTexts, Each colored ball in your kit corresponds to different atom or different group of atoms. Now the important point is, not to forget about the + sign. The relative arrangement of atoms is shown in the box. Determine the electron and molecular geometry of the produced molecules. Also state whether the neutral molecules are polar or nonpolar.

Solved 3. Draw Lewis Dot Structures For Each Of The Molec, The three dimensional shape or configuration of a molecule is an important characteristic. Draw sketches to predict the shape of a cif� ion and the shape of a clf5 molecule. 3 3 (c) name the geometry of the electron distribution about the oxygen atoms. Hydrazine ammonia, h nh h n nh acetic acid, h o methyl chloride, нсс cch h.
PART B Lewis Structures In the table provided, draw, Hybridization, bond angles and polarity draw lewis dot structures in your notebook for pcls, cio2 , sibr4, of2, and bros (structures obeying the octet rule). The following geometries may be. 3 molecule and the shape of the bf €ion. Ch3co2ch3 (h3ccooch3 one o atom attached to 2nd c atom; $$ \mathrm { cl } _ { 2 } \mathrm {.

5.) Draw Lewis dot structures for the following molecules, There are two lone pairs of electron. For each of the following molecules, draw a lewis structure and predict the molecular geometry, hybridization, overall polarity, and imfs of the molecule. These diagrams tell us that the f 2 molecule has a single bond, the co 2 molecule has two double bonds, and the hcn molecule has one single bond plus.

Draw A Threedimensional Structure For Each Compou, Determine the approximate bond angles. Also state whether the neutral molecules are polar or nonpolar. Learn this topic by watching 3d hybrid orbital drawings concept videos. Remember that, there are total of thirteen electron pairs. Hydrazine ammonia, h nh h n nh acetic acid, h o methyl chloride, нсс cch h oh carbon dioxide, oco hydrogen cyanide, hcn