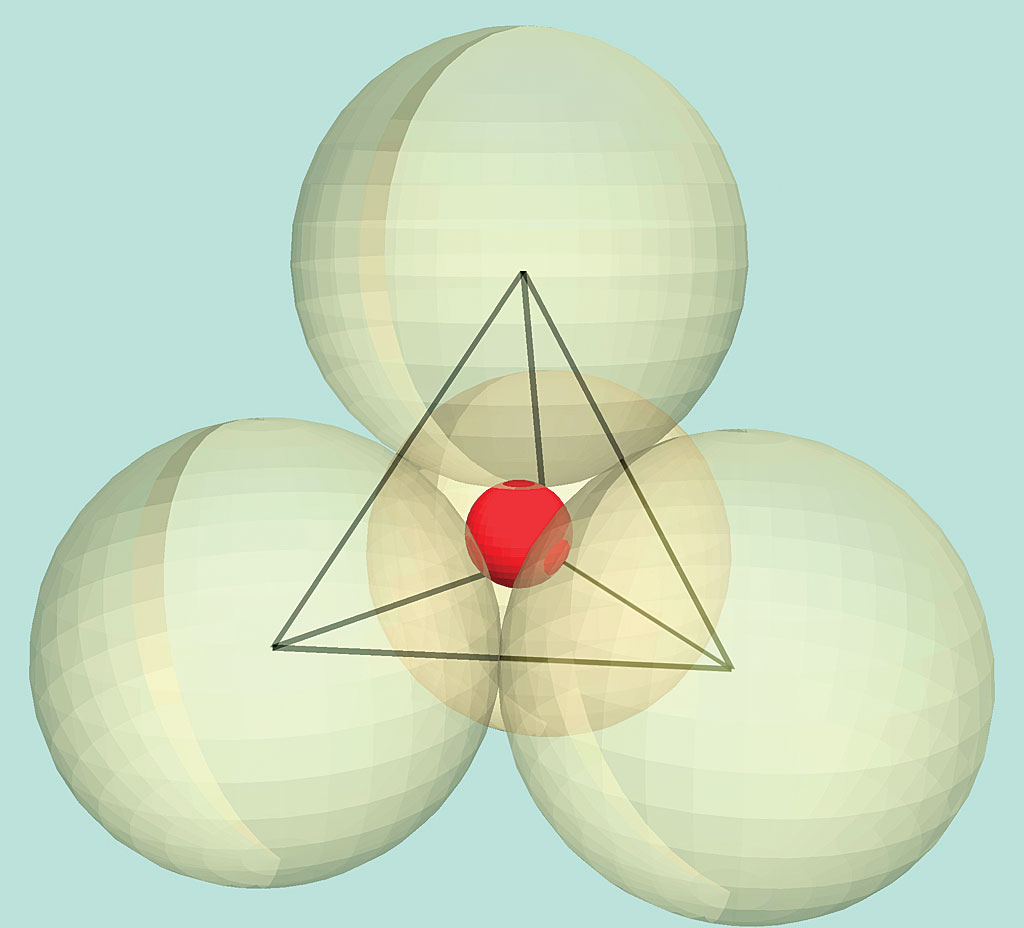
C) at right, a geometric shorthand. What is the most abundant mineral in earth's crust?
Draw A Sketch Of The Silicon Oxygen Tetrahedron, Since the one silicon cation has a +4 charge and the two oxygen anions each have a −2 charge, the charge is balanced. 3.please draw a simple crystal structure for each of the following silicate systems (see fig. List the eight most common elements in earth�s crust in.
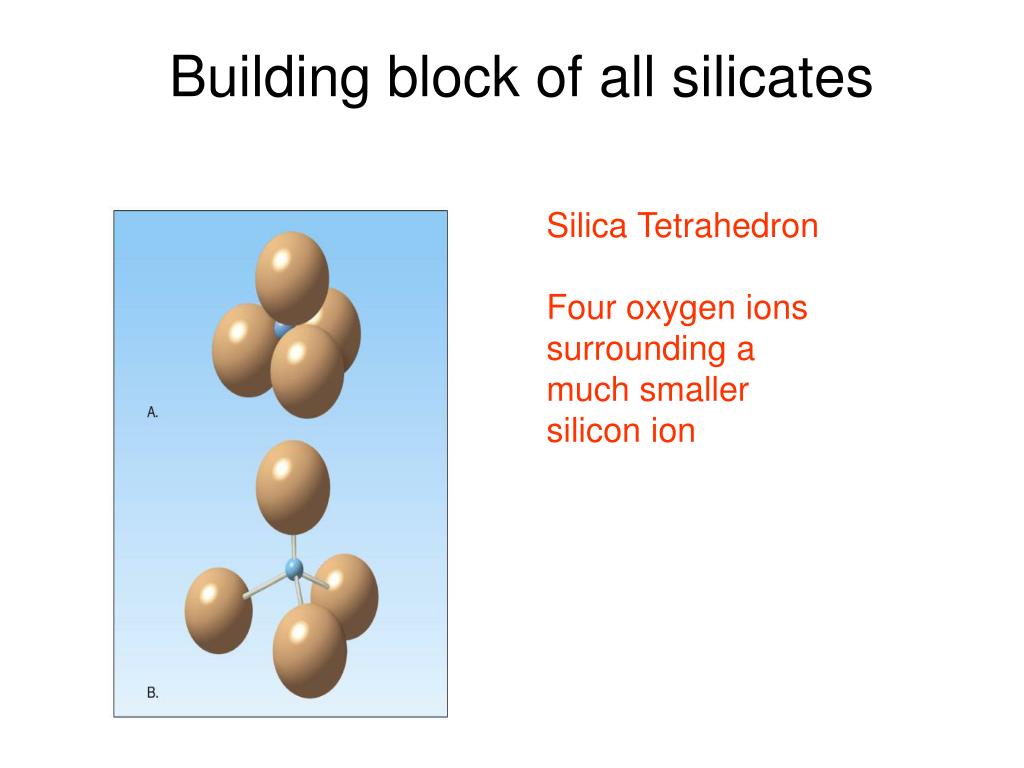
Three ways of drawing the silica tetrahedron: Figure 13.9 refer to figure 13.9. The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom. In silicate minerals, these tetrahedra are arranged and linked together in a variety of ways, from single units to complex frameworks (figure 2.9).
1NOT Mapping Our World
C) at right, a geometric shorthand. List the eight most common elements in earth�s crust in. It is composed of a central silicon cation (si 4+) bonded to four oxygen atoms that are located at the corners of a regular tetrahedron. C) at right, a geometric shorthand. These are arranged such that planes drawn through the oxygen atoms form a tetrahedron (figure 2.6). The geometric figure drawn around this arrangement has four sides, each.
![]()
PPT Prentice Hall EARTH SCIENCE PowerPoint Presentation, The chemical structure of silica forms a tetrahedron. 3.please draw a simple crystal structure for each of the following silicate systems (see fig. C) at right, a geometric shorthand. Since the one silicon cation has a +4 charge and the two oxygen anions each have a −2 charge, the charge is balanced. A variety of silicate minerals can be identified.

1NOT Mapping Our World, A) at left, a ball & stick model, showing the silicon cation in orange surrounded by 4 oxygen anions in blue; What is the most abundant mineral in earth�s crust? This is a fundamental component of most silicates in the earth’s crust. The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms.

Minerals, These are arranged such that planes drawn through the oxygen atoms form a tetrahedron (figure 2.6). The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom. Iodine is in period 5 on the periodic table. It is composed of a central silicon cation (si 4+) bonded to four.

Geophysics and plate tectonics It�s a natural universe, Silicates are minerals that contain siliconatoms bonded to oxygenatoms. Three ways of drawing the silica tetrahedron: This is a fundamental component of most silicates in the earth’s crust. Draw a sketch of an oxygen atom. Explain the difference between the terms silicon and silicate.
![]()
Symbol and electron diagram for Silicon Royalty Free Vector, The basic building block for all silicate minerals is called a tetrahedron, where one siliconatom is bonded to 4 oxygenatoms (figure 3.6). The chemical structure of silica forms a tetrahedron. (1) ball & stick, (2) space filling and (3) polyhedral. A variety of silicate minerals can be identified by the way that the tetrahedra links differ, also by the cations.

Geology 1403 Physical Geology The Silicate Minerals, These are arranged such that planes drawn through the oxygen atoms form a tetrahedron (figure 2.6). This is a fundamental component of most silicates in the earth�s crust. And then sketch, label, and explain how one tetrahedron can join with another (4.7a). Sketch and label a covalent bond and an ionic bond (you do not need to draw the metallic.

The Silicate Minerals Earth Science Visionlearning, C) at right, a geometric shorthand. Silicate minerals also often contain other elements, such as calcium, iron, and magnesium. Mg +2 combines to form the stable mineral forsterite. The basic building block for all silicate minerals is called a tetrahedron, where one siliconatom is bonded to 4 oxygenatoms (figure 3.6). Use the drawing as a model for a single silica.

Rocks & Minerals, The chemical structure of silica forms a tetrahedron. A firm produces hula hoops in a perfectly competitive market and currently produces and sells 100 per week. And then sketch, label, and explain how one tetrahedron can join with another (4.7a). Figure 13.9 refer to figure 13.9. It is composed of a central silicon cation (si 4+) bonded to four oxygen.

5.4 Silicate Minerals Physical Geology, First University, B) at center, a space filling model; These are arranged such that planes drawn through the oxygen atoms form a tetrahedron (figure 2.6). Three ways of drawing the silica tetrahedron: What is the most abundant mineral in earth�s crust? 3.please draw a simple crystal structure for each of the following silicate systems (see fig.
PPT Classification of Minerals PowerPoint Presentation, Approximately 50 percent ionic… read more A) at left, a ball & stick model, showing the silicon cation in orange surrounded by 4 oxygen anions in blue; Iodine is in period 5 on the periodic table. Sketch and label a covalent bond and an ionic bond (you do not need to draw the metallic bond or the intermolecular force). This.

Silicones 1. Silicate Structures Chemistry LibreTexts, Sketch and label a covalent bond and an ionic bond (you do not need to draw the metallic bond or the intermolecular force). At this production quantity of 100 per week, society would be best served. A firm produces hula hoops in a perfectly competitive market and currently produces and sells 100 per week. • sketch the crust, mantle, and.

PPT Elements Introduction PowerPoint Presentation, free, Refer to the information provided in figure 13.9 below to answer the question (s) that follow. C) at right, a geometric shorthand. Silicates are minerals that contain siliconatoms bonded to oxygenatoms. Figure 13.9 refer to figure 13.9. 3.please draw a simple crystal structure for each of the following silicate systems (see fig.

Mr. Cox�s Website, The basic structural unit of all silicate minerals is the silicon tetrahedron in which one silicon atom is surrounded by and bonded to (i.e., coordinated with). The geometric figure drawn around this arrangement has four sides, each. There is no need for aluminum or any of the other cations such as sodium or potassium. Draw a sketch of an oxygen.
![]()
Siliconoxygen tetrahedron mineralogy Britannica, Use the drawing as a model for a single silica tetrahedron (dot = oxygen) isolated silica tetrahedron (no oxygens shared) the general formula for this is sio4 (one si for each four o atoms) paired silicate tetrahedra (one oxygen shared. Figure 13.9 refer to figure 13.9. There is no need for aluminum or any of the other cations such as.

Silicates And Carbonates, There is no need for aluminum or any of the other cations such as sodium or potassium. List the eight most common elements in earth�s crust in. Refer to the information provided in figure 13.9 below to answer the question (s) that follow. Mg +2 combines to form the stable mineral forsterite. What is the difference between an atom and.

Unit 1 Introduction, Minerals, Igneous Rocks, and, Since the one silicon cation has a +4 charge and the two oxygen anions each have a −2 charge, the charge is balanced. These are arranged such that planes drawn through the oxygen atoms form a tetrahedron (figure 2.6). A firm produces hula hoops in a perfectly competitive market and currently produces and sells 100 per week. Mg +2 combines.

See the Electron Configuration of Atoms of the Elements, A firm produces hula hoops in a perfectly competitive market and currently produces and sells 100 per week. And then sketch, label, and explain how one tetrahedron can join with another (4.7a). The central atom is silicon. Explain the difference between the terms silicon and silicate. These are arranged such that planes drawn through the oxygen atoms form a tetrahedron.

Geophysics and plate tectonics It�s a natural universe, Draw a sketch of an oxygen atom. In silicate minerals, these tetrahedra are arranged and linked together in a variety of ways, from single units to complex frameworks (figure 2.9). Approximately 50 percent ionic… read more A firm produces hula hoops in a perfectly competitive market and currently produces and sells 100 per week. Three ways of drawing the silica.

Learning Geology Mineral Classification, Draw a sketch of an oxygen atom. A firm produces hula hoops in a perfectly competitive market and currently produces and sells 100 per week. What is the difference between an atom and an ion? The basic structural unit of all silicate minerals is the silicon tetrahedron in which one silicon atom is surrounded by and bonded to (i.e., coordinated.

PPT Rocks are aggregates of minerals. Many are silicate, Using this image, label the atoms with the appropriate elements to make the silica tetrahedron What is the most abundant mineral in earth�s crust? B) at center, a space filling model; At this production quantity of 100 per week, society would be best served. Each tetrahedron is bonded to four other tetrahedra (with an oxygen shared at every corner of.

06 minerals rocks_forstudents, Silicates are minerals that contain siliconatoms bonded to oxygenatoms. Refer to the information provided in figure 13.9 below to answer the question (s) that follow. It consists of a central silicon atom surrounded by four oxygen atoms, with which the central atom bonds. Silicate minerals also often contain other elements, such as calcium, iron, and magnesium. Three ways of drawing.
Minerals and Mineral Groups Earth Science, The basic structural unit of all silicate minerals is the silicon tetrahedron in which one silicon atom is surrounded by and bonded to (i.e., coordinated with). Mg +2 combines to form the stable mineral forsterite. Approximately 50 percent ionic… read more Sketch and label a covalent bond and an ionic bond (you do not need to draw the metallic bond.

- rocks & minerals, What is silica tetrahedron composed of? • sketch the crust, mantle, and core and identify on the sketch the most common class of mineral in each of these three layers (4.10a). A firm produces hula hoops in a perfectly competitive market and currently produces and sells 100 per week. Silicates are minerals that contain siliconatoms bonded to oxygenatoms. What is.

Silicates Boundless Chemistry, Since the one silicon cation has a +4 charge and the two oxygen anions each have a −2 charge, the charge is balanced. Using this image, label the atoms with the appropriate elements to make the silica tetrahedron Three ways of drawing the silica tetrahedron: What is silica tetrahedron composed of? What is the most abundant mineral in earth�s crust?

3.1 Silicate Mineral Groups A Practical Guide to, Figure 13.9 refer to figure 13.9. Refer to the information provided in figure 13.9 below to answer the question (s) that follow. Three ways of drawing the silica tetrahedron: Sketch and label a covalent bond and an ionic bond (you do not need to draw the metallic bond or the intermolecular force). Approximately 50 percent ionic… read more