• most molecules/ consist of one central atom bonded to 2, 3 or 4 other atoms. Valence errors will be highlighted (if that option is enabled).
How To Draw A Valence Bond Sketch, The second step is to valence electron to the two chlorine atoms, and the final step is to combine the step1 and step2 to get the Click and drag an object. After determining the center atom and sketch of pcl 5 molecule, we should start to mark lone pairs on atoms.
Draw the nucleus of an atom. In chemistry, valence bond (vb) theory is one of two basic theories—along with molecular orbital (mo) theory—that use quantum mechanics to explain chemical bonding. Count the electrons in the valence shell to check if the octet is completed. Try sketching the valence bond orbitals for ethene (ch,ch2) and ethyne (chch) to practice with double and triple bonds.
Solved Below Is A Valence Bond Theory Sketch Of Formaldeh
The second step is to valence electron to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the sf4 lewis structure. Click and drag an object. The electron pair geometry of so_2 is trigonal planar. Valence errors are highlighted (if that option is enabled). It is possible to draw up to 100 structures, because the chemsketch document can have 100 pages maximum. In chemistry, valence bond (vb) theory is one of two basic theories—along with molecular orbital (mo) theory—that use quantum mechanics to explain chemical bonding.

Valence Bond Theory PPTX, I make videos step by step for beginners. Sketch the overlap of the atomic orbitals involved in the bonds in o 2. Pi (t) bonds are formed when singly occupied p orbitals overlap above and below the internuclear axis. A nucleus is a dense and small region that contains the number of protons and neutrons of an atom. Now, we.

9.1 Valence Bond Theory Chemwiki, Bohr diagram is very interesting and easy to draw. Now, the central atom is. Sketch the overlap of the atomic orbitals involved in the bonds. Total # of valence electrons in mgbr 2 = 16; It is possible to draw up to 100 structures, because the chemsketch document can have 100 pages maximum.

Solved The Valence Bond Theory Describes A Covalent Bond, The first step is to sketch the lewis structure of the cacl2 molecule, to add valence electron around the calcium atom; Valence bond and molecular orbital models. The second step is to valence electron to the two chlorine atoms, and the final step is to combine the step1 and step2 to get the Customize structures to remove an atom, click.
[Solved] Use valence bond theory to explain the bonding in, Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine the total number of valence electrons to be depicted in the lewis diagram. To link two existing atoms, click on one then drag the cursor to the other. The simplest way is to paste carbon dioxide into the canvas. Electrons, drawn as.
[Solved] Use valence bond theory to explain the bonding in, Electrons, drawn as dots) or bonds (shared electrons, drawn as lines). Sketch the overlap of the atomic orbitals involved in the bonds. Remember that, there are total of 20 electron pairs to mark as bonds and lone pairs in overall molecule. Triple bonds contain one o bond and two t bonds. Pi (t) bonds are formed when singly occupied p.
- The following drawing describes the valence bond, The total number of valence electrons. It is possible to draw up to 100 structures, because the chemsketch document can have 100 pages maximum. Use valence bond theory to explain the bonding in f 2, hf, and clbr. Draw the first electron shell. Draw a sketch of the molecule.
Reading and Writing MO Diagrams Chemistry LibreTexts, Add your electrons, the 2nd. In chemistry, valence bond (vb) theory is one of two basic theories—along with molecular orbital (mo) theory—that use quantum mechanics to explain chemical bonding. Now, we can draw a sketch of h 2 o to show how atoms are located in the molecule. Marvin will allow you to draw a bond between any two atoms.

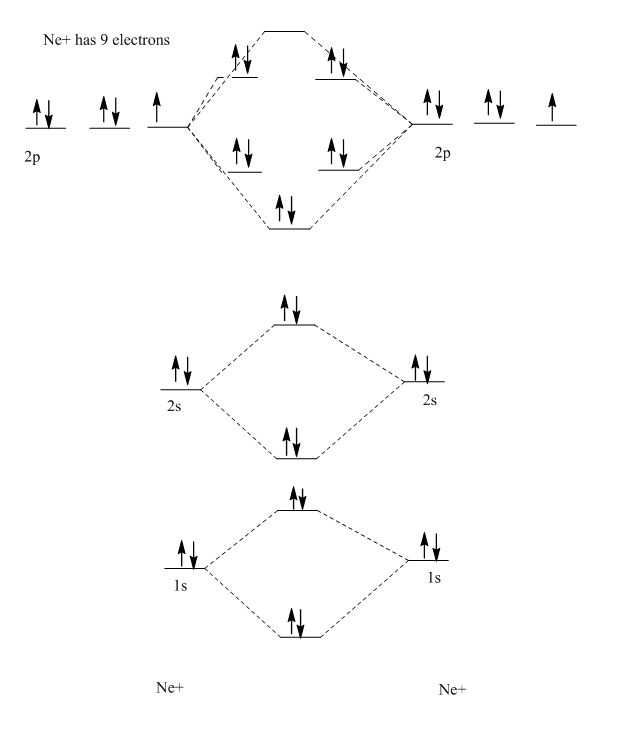
OneClass Draw The Valence Bond Lewis Structure of Ne2^+2, Draw a sketch of the molecule. (a) start with a skeleton structure. Sketch the overlap of the atomic orbitals involved in the bonds. Find the number of protons, electrons, and neutrons in the oxygen atom The first step is to sketch the lewis structure of the cacl2 molecule, to add valence electron around the calcium atom;

Covalent Bond Definition, Types, and Examples, The second step is to valence electron to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the sf4 lewis structure. Pi (t) bonds are formed when singly occupied p orbitals overlap above and below the internuclear axis. Click and drag an object. To link two existing atoms, click on one then.

Valence Bond Theory PPTX, Select a bond type using the bond toolbar button or by shortcut. A carbon atom is added at the other end of the bond. Use valence bond theory to explain the bonding in f 2, hf, and clbr. Try sketching the valence bond orbitals for ethene (ch,ch2) and ethyne (chch) to practice with double and triple bonds. Double bonds contain.

Valence Bond Theory PPTX, The electron pair geometry of so_2 is trigonal planar. Remember that, there are total of 20 electron pairs to mark as bonds and lone pairs in overall molecule. How are you i hope that you are fine and i also fine. Start from all valence orbitals empty, and count up, counting electrons. To draw a bond from a single atom,.
Solved Below Is A Valence Bond Theory Sketch Of Formaldeh, The simplest way is to paste carbon dioxide into the canvas. Draw the structures (with and without counter ions). Now, we can draw a sketch of h 2 o to show how atoms are located in the molecule. Now, the central atom is. To link two existing atoms, select a bond type, click on one then drag the cursor to.

Tang 06 valence bond theory and hybridization, A nucleus is a dense and small region that contains the number of protons and neutrons of an atom. (b) attach the hydrogen atoms. Draw orbital diagrams of each atom. The second step is to valence electron to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the sf4 lewis structure. How.

Complete Explanation of Valence Bond Theory YouTube, Start from all valence orbitals empty, and count up, counting electrons. Sketch the overlap of the atomic orbitals involved in the bonds. Select a bond type using the bond toolbar button or by shortcut. Procedure for drawing lewis structures 1. The second step is to valence electron to the four fluorine atoms, and the final step is to combine the.

C2h4 Dot Diagram, Remember that, there are total of four electron pairs. Double bonds contain one o bond and one ↑ bond. After determining the center atom and sketch of h 2 o molecule, we should start to mark lone pairs on atoms. Select a bond type using the bond toolbar button or by shortcut. Now, we can draw a sketch of h.

What�s the difference between a formula unit and a, To draw a bond from a single atom, simply click the atom. Draw orbital diagrams of each atom. The two c atoms (least electronegative) will be the central atoms, with the n attached to one of the carbons. Total # of valence electrons in mgbr 2 = 16; The bond order can be interpreted from mo diagrams using the following.
Solved Below Is Valence Bond Theory Sketch Of Formaldehyd, Now, we can draw a sketch of h 2 o to show how atoms are located in the molecule. The central atom, s, has three groups bonded to it, two oxygen atoms and a lone pair. After determining the center atom and sketch of h 2 o molecule, we should start to mark lone pairs on atoms. Marvin allows you.
Solved 5) Draw The Valence Bond Model Of Maleic Acid Diag, The electron pair geometry of so_2 is trigonal planar. The bond order can be interpreted from mo diagrams using the following formula: The first step is to sketch the lewis structure of the cacl2 molecule, to add valence electron around the calcium atom; After determining the center atom and sketch of pcl 5 molecule, we should start to mark lone.

CHEMISTRY 11 ELECTRONIC STRUCTURE DRAWING ELECTRON DOT, To link two existing atoms, click on one then drag the cursor to the other. Try sketching the valence bond orbitals for ethene (ch,ch2) and ethyne (chch) to practice with double and triple bonds. A nucleus is a dense and small region that contains the number of protons and neutrons of an atom. Now, we can draw a sketch of.

Solved Valence Bond Theory The Skeletal Structure For Met, Start from all valence orbitals empty, and count up, counting electrons. Electrons, drawn as dots) or bonds (shared electrons, drawn as lines). Select a bond type using the bond toolbar button or by shortcut. Add up the total number of valence electrons that each atom contributes to the. After determining the center atom and sketch of pcl 5 molecule, we.

2.1 Valence Bond Theory Chemistry LibreTexts, Steps to draw the bohr model of oxygen atom. Click atom properties to change the valence, charge, and isotope of an atom. Linus pauling became the champion of. Double bonds contain one o bond and one ↑ bond. Bohr diagram is very interesting and easy to draw.
[Solved] Use valence bond theory to explain the bonding in, To draw a bond from a single atom, simply click the atom. In the structure to replace it. Valence errors are highlighted (if that option is enabled). Here, we will draw the bohr diagram of the oxygen atom with some simple steps. Determine electrons involved in bonding.

Valence Bond Theory MCC Organic Chemistry, Marvin will allow you to draw a bond between any two atoms in the molecule. The central atom, s, has three groups bonded to it, two oxygen atoms and a lone pair. To link two existing atoms, click on one then drag the cursor to the other. Procedure for drawing lewis structures 1. It is drawn as the molecular geometry.

Valence Bond Theory Chemwiki, The second step is to valence electron to the four fluorine atoms, and the final step is to combine the step1 and step2 to get the sf4 lewis structure. Combine each atom with a single bond to the central atom by contributing one electron from each atom for the bond. In this step, we have to draw a small circle.

CHEMISTRY 11 ELECTRONIC STRUCTURE DRAWING ELECTRON DOT, Pi (t) bonds are formed when singly occupied p orbitals overlap above and below the internuclear axis. Find the number of protons, electrons, and neutrons in the oxygen atom Combine each atom with a single bond to the central atom by contributing one electron from each atom for the bond. Here, we will draw the bohr diagram of the oxygen.