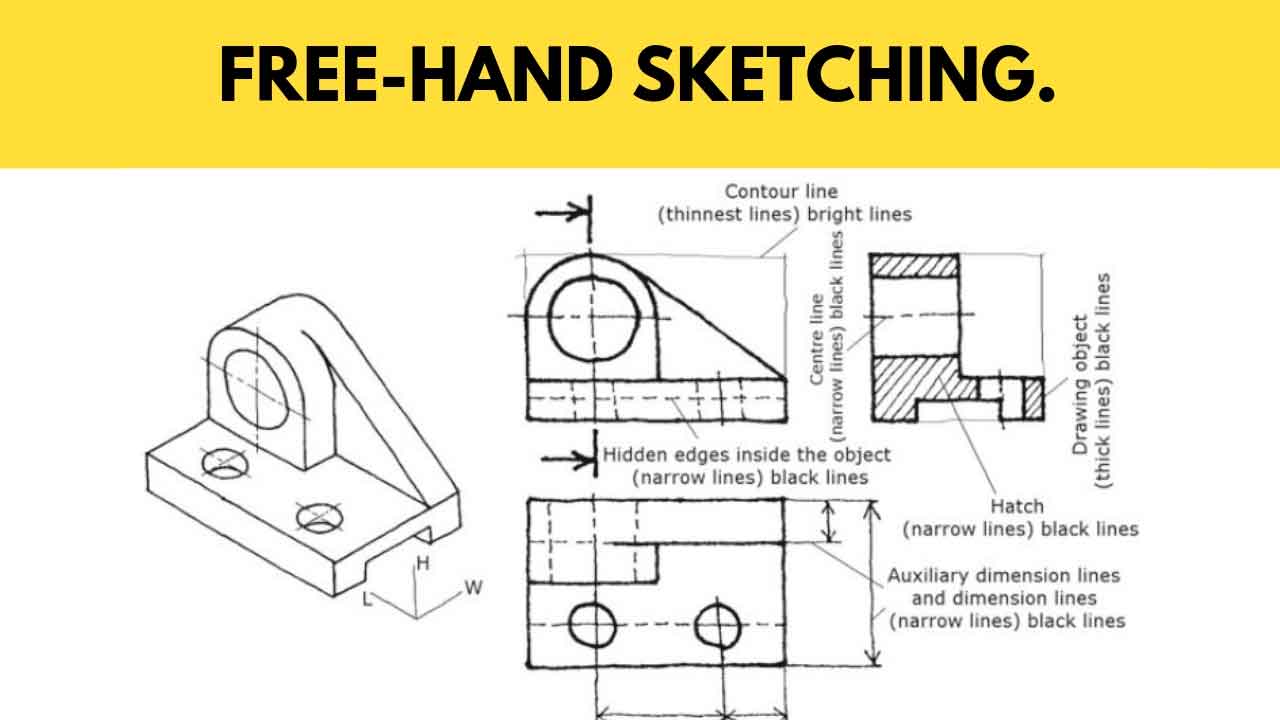
An illustration of the shape and relative size of 1s, 2s and 2p orbitals. 71 info 3 sketches mr doob with video tutorial
Sketch A Drawing For 1S And 2P, Valence electrons (ves) 8 o. (b) what is meant by a node? (9 points) sketch the 2s σ *, 2p z σ b, and 2p x π * molecular orbitals and indicate the phase of each lobe and all nodal planes perpendicular to the bond axis.
To draw lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table below. You can rotate the sketch for a better view of the orbital by dragging the slider with your mouse. At a distance of 3.0 å, s(1s,1s) is only 0.003. 41 info how to sketch for 2 hours sims 4 with video tutorial;
sketch 1s 2s 2p orbitals using the same scale for each
Sketch the 1s and 2p orbitals. Σ *2s, σ *2p z, π *2p x, π *2p y. Free online drawing application for all ages. Sketch the 1s and 2p orbitals. Between the compact 1s and diffuse 2s/2p orbitals and because the 2s and 2p orbitals are some 20 ev higher in energy than the 1s orbitals (8). The overlap integral between an h 1s orbital and a h2p orbital on nuclei separated by a distance r is s =.

Draw shapes of 2s and 2p orbitals class 11 chemistry CBSE, The contour plot of wavefunction (ψ) shown below is of one of the p orbitals: How would the 4d orbitals differ from the 3d orbitals? 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 _____ c. Sketch the 1s and 2p orbitals. Valence electrons in o3 =.

Sketch the 3d orbitals. How do the 4d orbi… Clutch Prep, Draw shapes of ‘2s’ and ‘2p’ orbitals. (c) how many radial nodes and angular nodal surfaces does each orbital have? Sketch the 2px and 2py orbitals (around the same nucleus, please). (9 points) sketch the 2s σ *, 2p z σ b, and 2p x π * molecular orbitals and indicate the phase of each lobe and all nodal planes.

Solved Part A Choose The Sketches Of The Following Orbita, Are the p electrons in the same orbital? 1s l = 0 1s it is also possible to show the orbital as a simple loop. Sketch 1s 2s 2p orbitals using the same scale for each 2 see answers advertisement advertisement pstnonsonjoku pstnonsonjoku as the orbital becomes farther from the nucleus, its energy increases and it becomes larger and more.

sketch 1s 2s 2p orbitals using the same scale for each, For the bonding mo, there is more electron density around the oxygen. An orbital box diagram can be written as well. Draw radial probability density and radial probability distribution curves for 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p. Using spectroscopy, the energy levels of these molecular orbitals are determined experimentally. Sketch the 2px and 2py orbitals (around the same.

How to draw Jordan 1s YouTube, See the answer see the answer see the answer done loading. Here is a sketch of a 2p, orbital: 4f 4d 4s 4p 3d 3p 3s 2p 2s 1s 8. Sketch 1s 2s 2p orbitals using the same scale for each 2 see answers advertisement advertisement pstnonsonjoku pstnonsonjoku as the orbital becomes farther from the nucleus, its energy increases and.

Draw the shape of 1s and 2s orbitals Brainly.in, An orbital is a region in space where there is a high probability of finding an electron. Briefly, drawings reflect the probability density distribution for the wave function outputs for a specific orbital with quantum numbers n, l, m_l and m_s. And we have the two p z. Since the antibonding effect is approximately Reading the periodic table from left.

Solved Sketch The 1s And 2p Orbitals. How Do The 2s And 3, 1s 2 2s 2 2p 4. Σ *2s, σ *2p z, π *2p x, π *2p y. 1s^2 2s^2 2p^5 _____ b. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 _____ c. What element is represented by the following ground state electron configuration?

MANCHI on Instagram “速畫練習 nike Quick Sketch practice, Click here👆to get an answer to your question ️ state significance of ψ and ψ^2. Free online drawing application for all ages. The contour plot of wavefunction (ψ) shown below is of one of the p orbitals: The 4 main types of orbitals are named ‘s’, ‘p’, ‘d’ and. Be sure your diagrams also indicate the relative electron density around.

Sketch 2p! Japan Hetalia japan, Japan, Hetalia, 1s l = 0 1s it is also possible to show the orbital as a simple loop. How would the 2s and 3p orbitals differ from the 1s and 2p orbitals? So if i�m looking at one ass one as is closest to the nucleus and it is c What element is represented by the following ground state electron configuration?.

What is an orbital Draw the shapes of the 1s 2s 2px class, And if we look at the toupee orbital�s x, y and z axes and we would have lobes like so this would be the two p x, and then we would have the two p y. Based on the amount of orbital overlap, the relative changes in energy differ going from the atomic orbital to the molecular orbital. And again.

A.1. Molecular Orbital Theory Chemistry LibreTexts, Sketch 1s 2s 2p orbitals using the same scale for each 2 see answers advertisement advertisement pstnonsonjoku pstnonsonjoku as the orbital becomes farther from the nucleus, its energy increases and it becomes larger and more diffuse. Draw this function for the 1s, 2s ,3s, 2p, 3p and 4 p orbitals in a hydrogen atom. This sketch is about 800 pm.

Draw shap of 2s and 2p orbital Brainly.in, 1s^2 2s^2 2p^6 3s^2 3p^6 4s^3 3d^10 4p^6 5s^2 4d^10 5p^4 _____ d. Before doing the calculation below, sketch how the overlap between an 1s orbital and a 2p orbital can be expected to depend on their separation. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 _____ c. Based on the amount of orbital overlap, the relative changes in energy differ going.

Sketch 1s 2s 2p Orbitals Coloring Pages, Define the concept of electron screening (shielding) in your own words. These orbitals are of 4 types and are distinguished on the basis of their geometries. What element is represented by the following ground state electron configuration? The order of increasing energy of molecular orbitals obtained by combination of 1s, 2s and 2p orbitals of two atoms is → σ1s,.

Como Dibujar The Odd 1s Out 😀 Dibujos Para Dibujar Faciles, Draw an energy diagram that illustrates the increase in energy of the sublevles found in n=1 out to n=4. To draw lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table below. We�re going to compare different sub levels, a different energy levels. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^3 3d^10.

Solved Here Is A Sketch Of A 2p, Orbital This Sketch Is, 79 info how to sketch 1s 2s and 2p orbitals with video tutorial; As the internuclear distance increases, the overlap integral falls off sharply; How do the 2s and 3p orbitals differ from the 1s and 2p orbitals?. So if i�m looking at one ass one as is closest to the nucleus and it is c Sketch the 1s and.

How to draw Odd 1�s Out step by step YouTube, This sketch is about 800 pm wide. Sketch the 2px and 2py orbitals (around the same nucleus, please). I�m going to draw this two dimensionally, but, uh, it would be in three dimensions a sphere. Draw this function for the 1s, 2s ,3s, 2p, 3p and 4 p orbitals in a hydrogen atom. And again these would be in three.

Draw shapes of 2s and 2p orbitals class 11 chemistry CBSE, Draw shapes of ‘2s’ and ‘2p’ orbitals. Σ *2s, σ *2p z, π *2p x, π *2p y. Sketch 1s 2s 2p orbitals using the same scale for each 2 see answers advertisement advertisement pstnonsonjoku pstnonsonjoku as the orbital becomes farther from the nucleus, its energy increases and it becomes larger and more diffuse. 79 info how to sketch 1s.

The Odd 1s Out Drawing YouTube, They are called contour representations. So if i�m looking at one ass one as is closest to the nucleus and it is c 65 info jhope sketch drawing with video tutorial; To draw lewis structure, we need to figure out the number of valence electrons in individual atoms as shown in the table below. The order of increasing energy of.

Shapes of the 3d orbitals in 3D — ChemTube3D Schematic, 1s here are some boxes for you to practice drawing s orbitals in, although you do not really need boxes. The contour plot of wavefunction (ψ) shown below is of one of the p orbitals: So the 1st 2 we�re gonna look at is one ass and two p. The overlap integral between an h 1s orbital and a h2p.

Cool Drawing Of 2p America Pt. 1 Hetalia, 2p america, Sketch 1s 2s 2p orbitals using the same scale for each 2 see answers advertisement advertisement pstnonsonjoku pstnonsonjoku as the orbital becomes farther from the nucleus, its energy increases and it becomes larger and more diffuse. We�re going to compare different sub levels, a different energy levels. The 4 main types of orbitals are named ‘s’, ‘p’, ‘d’ and. And.

How to Draw The Odd 1s Out StepbyStep, Before doing the calculation below, sketch how the overlap between an 1s orbital and a 2p orbital can be expected to depend on their separation. The contour plot of wavefunction (ψ) shown below is of one of the p orbitals: Briefly, drawings reflect the probability density distribution for the wave function outputs for a specific orbital with quantum numbers n,.

Draw shapes of 2s and 2p orbitals class 11 chemistry CBSE, The coordinate (x, y, and z) axes are also shown. Sketch the 1s and 2p orbitals. An illustration of the shape and relative size of 1s, 2s and 2p orbitals. Sketch the 2px and 2py orbitals (around the same nucleus, please). 1s 2 2s 2 2p 4.

Ingrays and 2p Romano drawing Hetalia Amino, How do the 2s and 3p orbitals differ from the 1s and 2p orbitals?. Before doing the calculation below, sketch how the overlap between an 1s orbital and a 2p orbital can be expected to depend on their separation. We�re going to compare different sub levels, a different energy levels. I�m going to draw this two dimensionally, but, uh, it.

HOW TO DRAW JORDAN 1 YouTube, You can rotate the sketch for a better view of the orbital by dragging the slider with your mouse. An illustration of the shape and relative size of 1s, 2s and 2p orbitals. Be sure your diagrams also indicate the relative electron density around each atom. I�m going to draw this two dimensionally, but, uh, it would be in three.

(Sketch) •2P!Hetalia• Amino, 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 _____ c. And if you are drawing his by hand, the loop does not have to be an exact circle. (9 points) sketch the 2s σ *, 2p z σ b, and 2p x π * molecular orbitals and indicate the phase of each lobe and all nodal planes perpendicular to the bond axis..